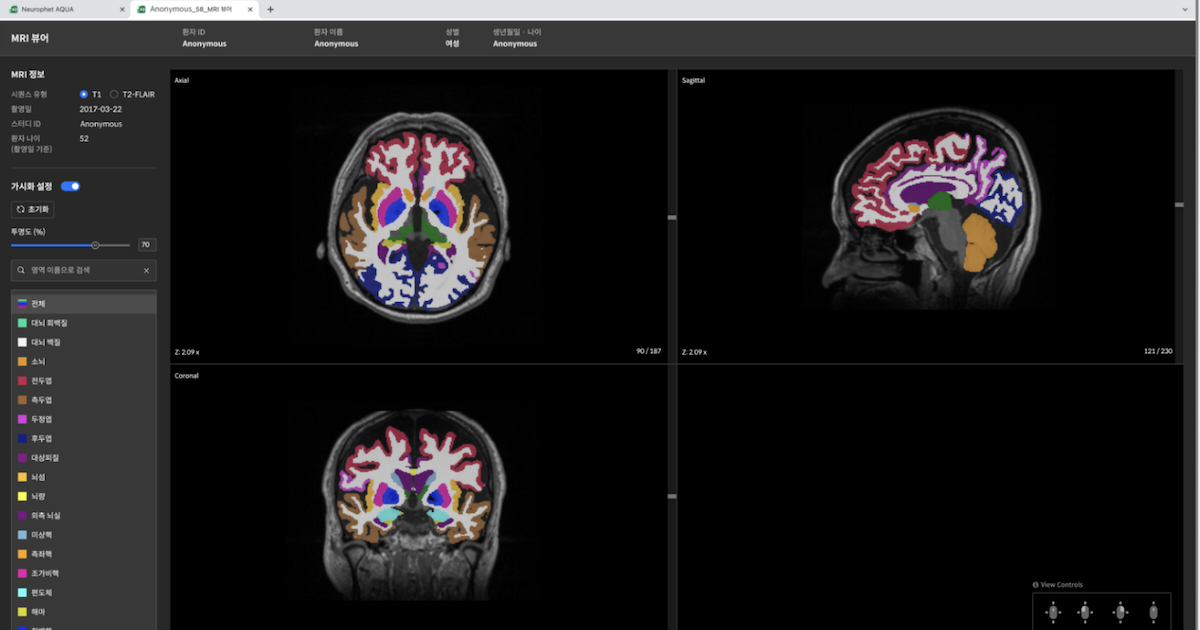
South Korean medical imaging AI company Neurophet has received second clearance for its brain image analysis software in the United States.
It obtained another 510(k) from the US Food and Drug Administration for the latest functionality on Neurophet Aqua that analyses multiple sclerosis and white matter hyperintensities using T2-FLAIR images.
The brain MRI scan-based analysis software, which uses AI to analyse brain atrophy observed in neurodegenerative diseases like Alzheimer’s disease, was first cleared by the US FDA in May last year.
Rival Vuno has obtained approval from the South Korean government for its AI-powered software analysing ECG data to identify hyperkalemia, a condition marked by high blood potassium levels.
The solution is the third in its line of ECG-based software to receive government regulatory approval. In May and August, Vuno secured clearances from the Ministry of Food and Drug Safety for DeepECG AMI (acute myocardial infarction) and LVSD (left ventricular systolic dysfunction).
Lunit, also a globally recognised medical imaging AI company from South Korea, has signed a supply deal with one of Mexico’s largest medical networks.
Salud Digna, which has over 230 clinics across Mexico and Central America, will deploy Lunit’s chest X-ray and mammography AI solutions.
The agreement also provides Lunit access to the network’s database of millions of deidentified 2D chest X-ray and mammography images, which would help refine and tailor its AI to the Latin American market.




