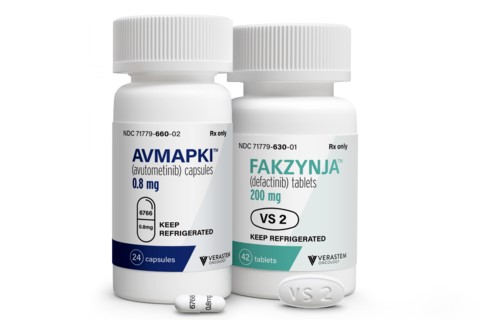
A rare type of ovarian cancer that grows slowly and responds poorly to chemotherapy now has its first FDA-approved treatment, a combination drug developed by Verastem Oncology to address a pathway that drives tumor progression.
The approval announced Thursday covers the treatment of low-grade serous ovarian cancer (LGSOC) driven by a KRAS mutation in adults who have received one prior systemic therapy. The Verastem drug, a pairing of two small molecules formulated as pills taken separately, will be marketed under the brand name Avmapki Fakzynja Co-Pack. Verastem expects its new product will launch next week.
Ovarian cancer comes in several different types. High-grade serous ovarian cancer (HGSOC) is the most common type, typically diagnosed in women between the ages of 40 and 60. Surgery and chemotherapy are the standard treatments for HGSOC. By contrast, LGSOC is rarer and often diagnosed in younger women, typically between the ages of 20 and 30 or between 50 and 60, according to Verastem. The company estimates that 6,000 to 8,000 women in the U.S. are living with LGSOC and 1,000 to 2,000 cases are diagnosed each year. Following a diagnosis, patients survive a median of 10 years. Surgery, chemo, and off-label use of hormone therapies are standard treatments for LGSOC. But this type of cancer does not respond well to those treatments and recurrence is common.
An estimated 70% of LGSOC tumors are driven by mutations in the MAPK pathway that regulates certain process such as cell growth. Mutations to genes such as RAS and MEK can dysregulate this pathway, contributing to the growth of cancer cells. The RAS family of genes was long thought to be undruggable. In 2021, Amgen’s Lumakras became the first FDA-approved KRAS inhibitor. This daily pill is approved for treating non-small cell lung cancer driven by KRAS G12C mutations.
The avutometinib component of the Verastem drug combination is a small molecule inhibitor of MEK proteins, proteins that when mutated can over activate the RAS/MAPK pathway. The defactinib component of Verastem’s drug is a small molecule inhibitor of two members of the FAK family of proteins that play roles in the growth, spread, and survival of cancer cells. According to Verastem, this combination is intended to provide a more complete blockade of the aberrant signaling that drives cancer growth and drug resistance of tumors that depend on the RAS/MAPK pathway.
Verastem evaluated the drug combination in an pivotal Phase 2 study that included 57 adults with measurable KRAS-mutated recurrent LGSOC. Participants in the open-label study had at least one prior systemic therapy, including a platinum-based chemotherapy. For the first three weeks of each four-week treatment cycle, avutometinib was taken twice weekly while defactinib was taken twice daily. Results showed a 44% overall response rate. The duration of response ranged from 3.3 months to 31.1 months. Serious complications reported from the study included eye, skin, and liver toxicities.
The regulatory decision announced Thursday is an accelerated approval made under a pathway that can get a drug to market faster for conditions with few or no available treatments. Drugs approved under this faster pathway must confirm their safety and efficacy in a larger Phase 3 study. That confirmatory study is underway; Verastem estimates to complete enrollment by the end of this year.
“The approval of avutometinib plus defactinib brings a much-needed therapeutic option to patients and establishes this combination as the new standard of care for women with recurrent low-grade serous ovarian cancer harboring a KRAS mutation,” Dr. Rachel Grisham, section head, ovarian cancer at Memorial Sloan Kettering Cancer Center, and global lead principal investigator of the Verastem drug’s clinical trials, said in a statement included in the company’s approval announcement. “I look forward to progressing the confirmatory Phase 3 trial, RAMP 301, where we look to continue to support the ongoing body of research of this combination in women with and without a KRAS mutation.”
In addition to potentially supporting full approval in LGSOC, Verastem said the Phase 3 study could also support expanding the drug combination to the treatment of LGSOC regardless of KRAS mutation status. Verastem said investigator-sponsored clinical trials are underway evaluating the drug combination in other gynecological cancers driven by MAPK pathway mutations. Mid-stage studies are also ongoing evaluating the drug combination in non-small cell lung cancer and pancreatic cancer.
As of the end of 2024, Verastem reported its cash position was $88.8 million. The company has since made moves to strengthen its finances in preparation for commercialization of Avmapki Fakzynja Co-Pack. In January, Verastem secured up to $150 million in debt financing; late last month, the company raised $75 million in a private placement of securities.
Photo by Verastem Oncology