Why is it so hard to estimate the value of orphan drugs indicated for the treatment of rare diseases? There are a variety of reasons, but a scoping review by Grand et al. (2024) provides a nice summary of these issues. Key challenges include small sample sizes for nearly all parameters and lack of data overall. More specifically, key issues identified in the paper include:
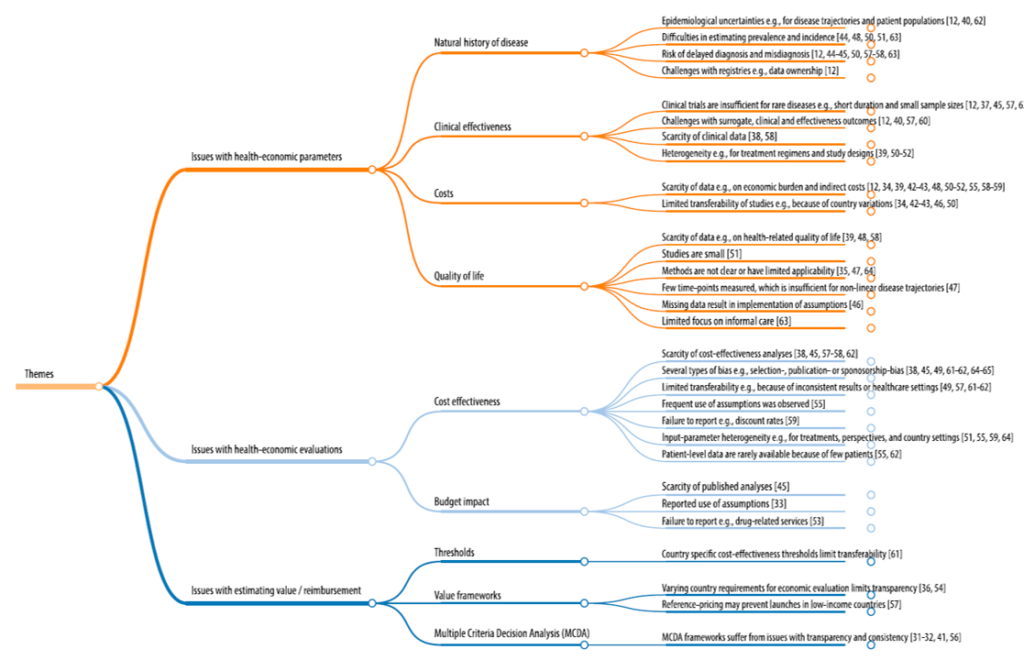
- Natural history of disease: Unclear epidemiological data (e.g., incidence, prevalence), unclear disease trajectories, frequent delayed diagnosis/misdiagnosis; challenges creating disease registries
- Clinical effectiveness. Trials are often short duration with small sample sizes; few or poorly validated surrogate endpoints; difficulty to compare treatments due to heterogeneity in treatment regimens and study designs.
- Costs. Limited data on economic burden of disease and indirect costs; transferability of cost inferences across studies challenging due to country variations
- Quality of life: Few studies on HRQoL and those that are conducted have small sample size; few disease-specific QoL metrics; HRQoL measured over limited time points making mapping non-linear disease trajectories difficult; limited focus on informal caregiving
- Cost effectiveness. Few previous studies; numerous biases (e.g., publication bias, sponsorship bias); limited transferability of CEA results due to inconsistent results of differences across health care settings; frequent use of assumptions; failure to report discount rate assumptions; input parameter heterogeneity; few patient level dat
- Budget impact. Few published BIM studies for any given disease; frequent use of unproven assumptions; failure to report drug-related care
- Value/reimbursement. Country-specific CEA thresholds for rare disease vary dramatically across countries; value framework requirements vary across country; reference pricing may prevent launches in low-income countries; use of MCDA can overcome some CEA limitations but produces others (e.g., transparency, consistency across treatments)
To overcome these barriers, the authors propose a number solutions including working directly with patient advocacy groups, creating disease registries, considering outcomes-based payment/risk sharing agreements. Working with patient advocates to collect data and creating disease registries is helpful; on the other hand, while outcomes-based payments would solve the uncertainty issue, they may be cost prohibitive as the largely fixed cost of setting up and administering these agreements may not be worth the cost if spread across very few patients.
You can read more details about challenges and opportunities in rare disease economic evaluations here.