Delays in drug approvals cost pharmaceutical firms money. These costs include both lost sales as well as additional cost if clinical trials need to span a longer duration. While these facts are not in doubt, a key question is how much do delays in approval cost firms?
A paper by Smith, DiMasi and Getz (2024) provides the answer.
…a single day equals approximately $500,000 in lost prescription drug or biologic sales, with daily prescription sales for infectious, hematologic, cardiovascular, and gastrointestinal diseases among the highest…The estimated direct daily cost to conduct a clinical trial is approximately $40,000 per day for phase II and III clinical trials, with those in respiratory, rheumatology, and dermatology having the highest relative daily direct costs.
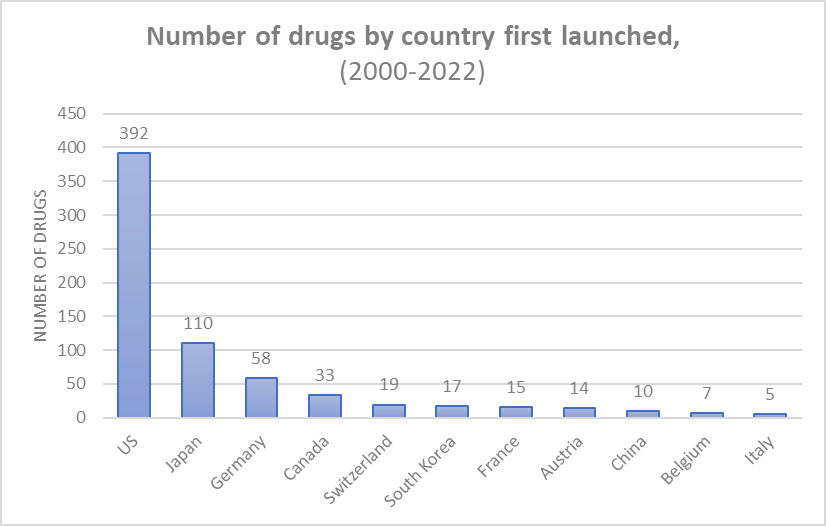
About 60% of drugs were first launched in the US. About 37.7% of drugs were “blockbuster” drugs with more than $1m of sales per day; 3.1% of drugs had >$7m of sales per day.
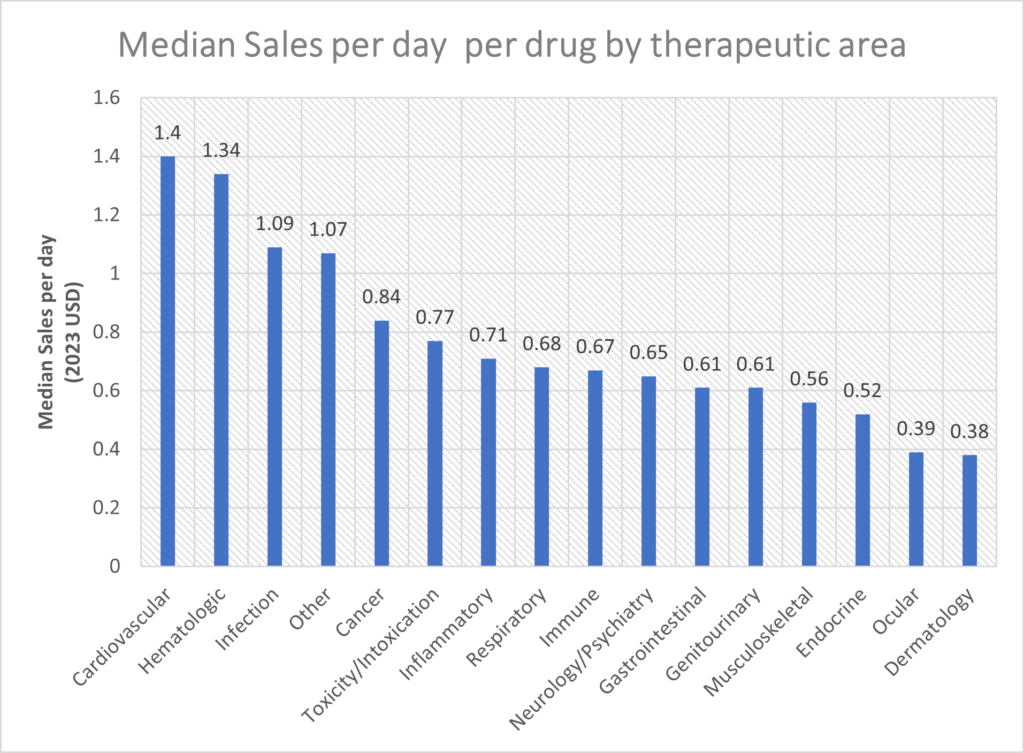
Cardiovascular, hematology, infection and oncology drugs had the highest sales per day based on median sales per day.
The average clinical trial cost $25 million and took 614.8 days to complete. As expected, later stage clinical trials are more expensive:
… phase III clinical trials have the highest daily cost at $55,716. Phase II clinical trials cost roughly half that amount at $23,737 per day (refer to Table 8). Phase IV and phase I trials have the lowest daily cost at $14,091 and $7,829 per day, respectively.
Methods
rug revenue data came from Cortellis database of drug sales for all drugs and biologics launched in 2000 or after. These data contain worldwide sales figures from company annual reports. As drug sales data were highly right-skewed the authors removed (i) COVID-19 drugs and vaccines and (ii) any drugs with sales >2 standard deviations from the mean
Drug development costs per day were estimated from a proprietary dataset developed and maintained by Tufts University’s Center for the Study of Drug Development (CSDD).






