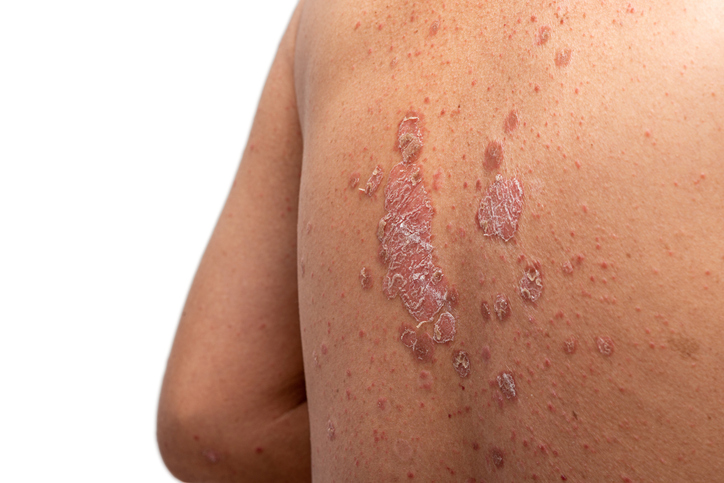
For a plaque psoriasis drug to succeed in a clinical trial, it doesn’t need to clear all of the redness and other signs of the inflammatory skin disease. But complete skin clearance is a benchmark many patients and physicians look for, and an experimental Takeda Pharmaceutical drug now has data from two pivotal studies showing it can reach that goal.
The once-daily pill, zasocitinib, helped more than half of patients in the studies achieve clear or almost clear skin at 16 weeks, Takeda said Thursday. With these results, the Tokyo-based drugmaker is planning to file submissions with the FDA and other regulatory agencies around the world in the coming year.
Zasocitinib (formerly known as TAK-279) is a small molecule inhibitor of TYK2, an enzyme that plays a role in signaling pathways associated with immune disorders such as plaque psoriasis. In 2022, Bristol Myers Squibb’s Sotyktu became the first FDA-approved medication in the TYK2 inhibitor drug class. The oral small molecule was approved for treating moderate-to-severe plaque psoriasis in adults. The promise of this target as a way to treat a range of immune disorders has sparked research and business development interest across the biopharmaceutical industry. Months after Sotyktu’s approval, Takeda reached a deal to acquire zasocitinib from Nimbus Therapeutics for $4 billion up front.
In an interview earlier this year, Andy Plump, Takeda’s president, research and development, said the differences between zasocitinib and Bristol’s Sotyktu show in Phase 2b data for the drugs. Activity of Bristol’s once-daily drug wanes over the course of the day, he said. By contrast, zasocitinib’s selectivity to TYK2 enables higher dosing to achieve inhibition of the target for an entire day.
“We get close to 100% inhibition across a 24-hour period, which is probably minimally three-to-four fold more than what you’re seeing with Sotyktu and the doses that they take forward,” Plump said. “That’s the difference. It’s a true best-in-class TYK2 inhibitor.”
The data reported Thursday are from two Phase 3 clinical trials that enrolled 1,801 patients combined. Each study compared the Takeda drug to a placebo and Otezla, an Amgen drug that is a standard plaque psoriasis treatment. Without releasing specific figures, Takeda said zasocitinib met the two main goals assessing its drug according to two skin clearance measures at 16 weeks. Responses were observed as early as week 4 and continued to increase through week 24. Furthermore, Takeda said the studies met additional secondary endpoints and showed the potential to deliver complete skin clearance.
Zasocitinib was generally well-tolerated, Takeda said. The most common adverse events reported through week 24 were upper respiratory tract infection, nasopharyngitis, and acne. No new safety signals were identified. Takeda plans to present more detailed results at upcoming medical meetings. The company added that it plans regulatory submissions to the FDA and other regulatory agencies in fiscal 2026, which starts on April 1.
Additional studies are ongoing for zasocitinib. One of them is testing the drug head to head against Sotyktu. BMS has broader plans for Sotyktu; the drug is under FDA review for psoriatic arthritis and Phase 2 tests are ongoing in lupus and Sjögren’s syndrome. But Sotyktu has fallen short in inflammatory bowel disease (IBD), and that’s one area where Takeda believes its drug can stand out.
When the BMS drug failed a Phase 2 test in ulcerative colitis in 2021, the company held out hope that a higher dose would lead to better results. Plump said it remains to be seen whether TYK2 inhibition can treat IBD, but Takeda’s ability to offer higher dosing of zasocitinib could achieve greater inhibition of the TYK2 target compared to other drugs in the class.
“Not only do we have a best-in-class profile in psoriasis but we think we have a shot in breaking through in IBD,” Plump said.
Photo: Getty Images






